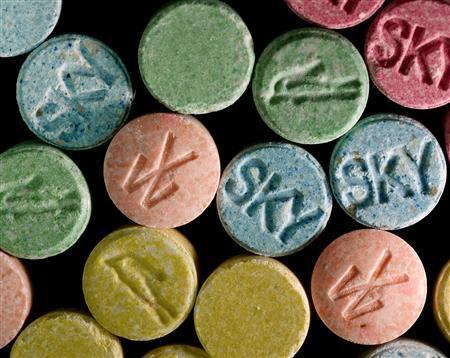
Ecstasy is known as a drug in the form of pills, usually circulated between teenagers, especially in parties. This is where it got its name “party drug”. Ecstasy was originally developed by Merck pharmaceutical company in 1912. In its original form, it was known as “MDMA.” It was used in 1953 by the US Army in psychological warfare tests, and then resurfaced in the 1960s as a psychotherapy medication to “lower inhibitions.”1 It wasn’t until the 1970s that MDMA started being used as a party drug.
It topped the list in the easrly 1980s of drugs that bring happiness in chemistry. It remained legal in 1984, and was being sold under the brand name “Ecstasy,” but by 1985, the drug had been banned due to safety concerns.
Ecstasy today contains a wide mixture of substances, from LSD, cocaine, heroin, amphetamine and methamphetamine, to rat poison, caffeine, dog deworming substances, etc.
What makes it Dangerous
What makes Ecstasy particularly dangerous is that a user never really knows what he/she is taking, due to the various and catchy labels used by drug dealers, according to Scientific websites.
The dangers are increased when users increase the dose seeking a previous high, not knowing they may be taking an entirely different combination of drugs.
Ecstasy most commonly comes in pill form but can also be injected and taken in other ways. Liquid Ecstasy is actually GHB, a nervous system depressant, a substance that can also be found in drain cleaner, floor stripper and degreasing solvents.
Approved in 2022?
Despite all that, this dangerous drug might be approved in 2020 by FDA. The Multidisciplinary Association for Psychedelic Studies (MAPS) would meet in spring with the US Food and Drug Administration (FDA) to plan the Phase 3 clinical trials that would make MDMA-assisted psychotherapy an approved treatment option.
FDA’s approval is hinged on the completion of the clinical trials, with Phase 3 slated to start in early 2017, and MAP’s clinical research team and lead therapists started to review applications from potential Phase 3 researchers from the almost 250 applicants it received from 10 countries, believing it could get the FDA approval for ecstasy.
In Phase 1 of the clinical trials, MAPS studied 20 PTSD patients and found that 83 percent no longer exhibited signs of the conditions two months after taking MDMA. Of the 20, patients, 12 were given ecstasy, but all received psychotherapy. Of the eight on placebo, 25 percent also showed an improvement.
Phase 2, about to end, has 136 participants who were given both ecstasy and psychotherapy. MAPS expects Phase 3 four to five years to complete, says Brad Burge, MAPS director of communications.
Besides PTSD, MDMA-assisted therapy is also used to treat autistic adults with social anxiety and anxiety linked with other conditions such as cancer and other life-threatening diseases.
Twitter: @greenareaen