This question originally appeared on Quora. Answer by Christopher VanLang, PhD.
A challenging question since scientists do have a potential path forwards to cure HIV infection, but they don’t make any practical sense since physicians shouldn’t resort to aggressive cures for HIV when the current anti-retroviral therapies (ART) are far less risky.
What we should be doing is focusing on small molecules and vaccines that allow us to eliminate latent HIV infection.
Based on several case studies, one could essentially cure HIV if they:
- Perform bone marrow transplants with a CCR5 deficient stem cell transplant similar to what was done for the Berlin patient.
- Use gene therapy to knockout key pathways in T-Cells namely CCR5.
- Use gene therapy to knockout key pathways in HIV.
- Add decoy cells to extract HIV from latency.
However, we would never realistically pursue any of these mechanisms of action in the near future unless an emergency requires it (like a bone marrow transplant to treat leukemia). The risks and costs of the above options outweigh the benefits compared to taking pills. While CRISPR/Cas9 is the new hot technology, it doesn’t make medical sense to apply a non-specific excision system using a Lentiviral vector in cells that will clear out in a couple of months thus requiring a new bolus of Lentiviral vectors unless you figure a way to target latently infected T-cells. Furthermore, these therapeutic strategies could not be successfully applied in third world countries where HIV is a more serious problem. We should look to more promising alternatives.
There are numerous challenges with developing a non-gene editing option to treat HIV. While patients are now effectively cured using ART using a combination of nucleoside analog reverse transcriptase inhibitors (NRTIs) and protease inhibitors, these treatments are effective in preventing further effects of HIV but do not remove the presence of HIV from the body. HIV is particularly difficult to remove because the virus can stay latent in the CD4+ T-cell genome for decades, and the constant threat of the reactivation of these latent HIV reservoirs require patients to strictly adhere to their ART regimens. Furthermore, drug resistant variants can evolve while in the latent phase. Despite several efforts of increased dosing with ART along with the introduction of anti-integrase drugs like raltegravir to prevent the reintroduction of HIV into the genome, the levels of latent HIV have not been affected indicating that the HIV is stably integrated into the resting memory CD4+ T-cells.
To enable long-term resistance of latent HIV reactivation without a life-time dependency on drugs, we should be focusing on two areas:
- Activation of HIV gene expression from resting CD4+ T-cells to reduce latent HIV
- Generation of broadly neutralizing antibodies using an anti-HIV vaccine.
REDUCE LATENT HIV
HIV resides in resting memory CD4+ T-cells which continuously regenerate and result in the life-long infection of HIV. Fortunately, there is a model Jurkat T-Cell line, J-Lat, which contains latent HIV. This model cell line can be used to further studies of the mechanisms of reactivation. Humanized mouse models and the SIV-macaque model are also useful models of latent HIV infection.
There are several remaining technical challenges to pursuing this path. First, it is still unclear how HIV establishes latent infection of these resting CD4+ T-cells since they have relatively low expressions of CCR5. Secondly, because of the difficulty of selectively activating latently infected cells from the HIV-free T-cells, global activation of resting T-cells using anti-CD3 antibodies or T-cell activation proteins like Interleukin-7 and Interleukin-2 have lead to severe adverse effects from cytokine release. Only 1 in 1 million resting T-cells are infected.
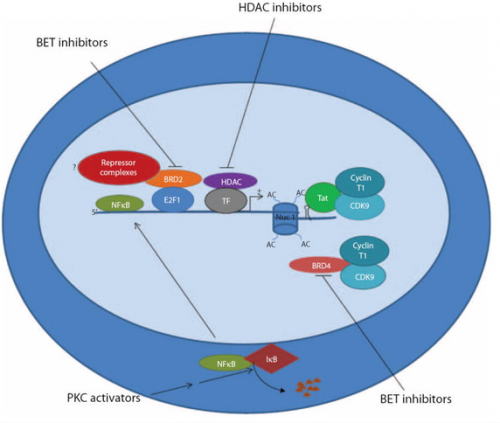
However, several studies on the epigenetics of HIV suppression has identified several pathways that suppress HIV activation.
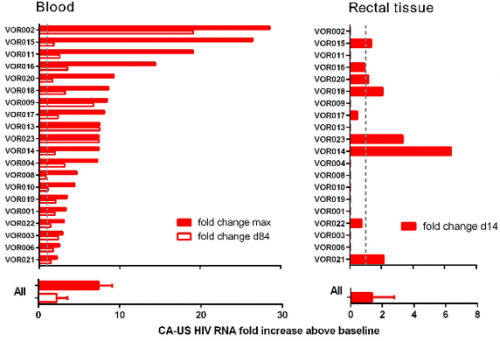
As such, groups have deferred to small molecule activators like bryostatin which is hypothesized to activate CD4. Certain clinical trials are exploring the use of the HDAC inhibitor vorinostat along with ART to activate HIV and transcriptional studies showed a significant change in viral RNA expression. At the moment, future clinical trials using vorinostat are mainly being investigated in high risk patients with HIV infection and cancer and this should serve as a strong proof of concept trial to test the effect of using HDAC inhibitors.
More likely, to see further use of these latent HIV T-Cell activators , we would need to perform cell-based screens that select for compounds that work only on infected cells and not on healthy cells. Until we have a good understanding of the risks, we wouldn’t be able to make sufficient progress in treating patients overseas. However, early Phase I work has suggested that we have the ability to drive out HIV from latency.
ANTI-HIV VACCINE
A huge amount of work has been put into the development of broadly neutralizing antibodies and the mechanisms to generate an immune response to both a preventative and therapeutic vaccine that would infer immunity to latent HIV.
The challenges behind HIV vaccine research is well documented on Quora and I highly recommend looking at Brian Dickerson’s answer to Why has developing an effective vaccine for HIV proven so difficult? Some of the major key points is that the exposed domain ENV of the HIV virus are rapidly evolving and are protected by several glycans which cover >70% of the protein.
A huge focus has been place on neutralizing antibodies to GP120, the CD4 binding site of HIV. Alternatively, one could develop broadly neutralizing antibodies towards GP41 which is better conserved. The RV144 “Thai trial” using the prime-boost HIV vaccine was determined to be largely ineffective despite successfully generating an immune response.
As outlined in [a 2013 study,] Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning, a huge number of the broadly neutralizing antibodies are directed towards the GP120 end of the ENV protein (the bottom). However, there are a few antibodies that will target the membrane-proximal external region (MPER) of GP41 notably the 10e8 antibody and the 35O22 antibody.
Read more on Medical Daily