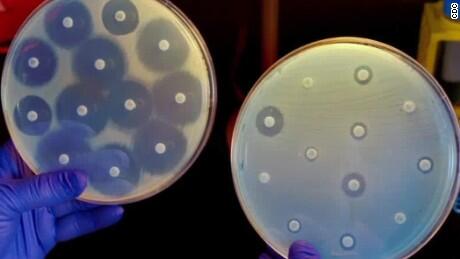
Human bodies are made up of human cells and microorganisms including mainly bacteria, in addition to viruses, parasites and fungi. The ratio was estimated in 1972 to be 10 microbes for every human cell, but recent reports state that the ratio is more likely to be one-to-one. Whether bacteria outnumber human cells or no, it is vital to know that bacteria are essential for digestion, synthesis of vitamins, boosting the immune system and providing protection against infections caused by pathogenic microorganisms. Only a small percentage of bacteria, less than 1 percent, are harmful to humans; and with the presence of antibiotics since the 1940s, bacterial infections can be easily combated. Yet, unfortunately, this is not the case in recent years, due to the emergence of a crisis named “Antibiotics Resistance”, which is at the moment the number one public enemy worldwide. In Lebanon, a study performed by Dr. George Araj, and his team, who is the director of Clinical Microbiology at the American University of Beirut (AUB), showed that there is an increase in the resistance to antibiotics in different types of bacteria, and this resistance is even to antibiotics that are extremely expensive and powerful, such as Carbapanems.
What is Antibiotics Resistance?
Bacteria outsmarted antibiotics through alterations in their genetic material. These new genes offer bacteria unique properties and allow them to defeat antibiotics. Although antibiotic resistance occurs naturally in bacteria over time, the misuse and overuse of antibiotics in human and veterinary medicine, accelerates this development. Therefore, the majority of bacteria now can prevent the entrance of antibiotics, pump antibiotics out or even produce chemicals that inactivate antibiotics.
According to the World Health Organization (WHO) October 2016 Fact Sheet, the worldwide increase in antibiotics resistance is remarkably dangerous to the extent that certain infections such as pneumonia, tuberculosis, blood poisoning and gonorrhea, are sometimes impossible to treat since the antibiotics that were previously effective treatments, are no longer helpful. A minimum of 700,000 people die annually as a result of drug-resistant infections, and a recent UK Commission study states that almost 10 million people will be killed annually, one person every three seconds, because of bacterial infections by 2050, if novel antibiotics are not manufactured.
Can Unusual Alternatives Resolve The Antibiotics Catastrophe?
Scientists are investing their time in searching for new antibiotics in unusual places, such as the saliva of “Komodo” dragons and algae-filled fur of “Sloths” in Panama. Others are testing antimicrobial peptides (AMPs) that are naturally present in all living organisms, in addition to their synthetic derivatives, since they both have antimicrobial and immunomodulatory properties that allow them to fight bacterial infections at many levels. Recently the synthetic derivative of Clavanin A, which is a naturally occurring AMP in the marine tunicate Styela clava, showed evidence of being an efficient novel alternative to the currently available antibiotics.
Also, scientists such as Ms. Kim Hardie, the microbiologist at Nottingham University, are considering the generation of antibiotics that instead of killing bacteria, prevent the communication of bacteria among each other which is vital for the initiation of a harmful attack. As per Ms. Hardie, experiments done up till now show positive outcomes and in 10 years we might have in the market antibiotics whose mechanism of action is based on this principle.
Ethnobotany: Even Future Remedies Are Plant-Based
The majority of available medications such as antibiotics, aspirin, morphine, codeine, pseudoephedrine and many anticancer drugs are derived from nature. And even when the antibiotics resistance crisis hit populations are over the globe, researchers went back to nature to search for alternatives. The enthnobotanist at Emory University in Atlanta, Cassandra Quave, is a leader in the development of new medicines from plants. Quave has merged the knowledge of traditional plant-based therapies with the advancement in laboratory work, and discovered candidates that can be potential future treatments for antibiotic-resistant bacterial infections. She discovered that extracts of “Elmleaf Blackberry” roots and Brazilian “Peppertree Berries” can prevent MRSA, antibiotic-resistant Staphylococcus bacteria, from forming biofilms which is the property that allows them to adhere to human tissues and contaminate medical devices. So, Quave proved that the kind of antibiotics that Ms. Kim Hardie is looking forward to, are actually found in nature.
To impede the evolution of antibiotics resistance, researchers should focus on searching for chemicals that inhibit bacteria from forming a critical mass, since once the latter happens, bacteria start releasing toxins, protecting themselves from drugs and exchanging genes that confer resistance to antibiotics. This approach will definitely make it impossible for bacteria to develop resistance, since these drugs are not directly killing bacteria.